Blog Post #2
(April 26, 2016)
Our last lecture of this unit presented us with two different types of graphs:
Heating and Cooling Curve: Shows where an element/compound will freeze/boil/melt.
 |
| Add caption |
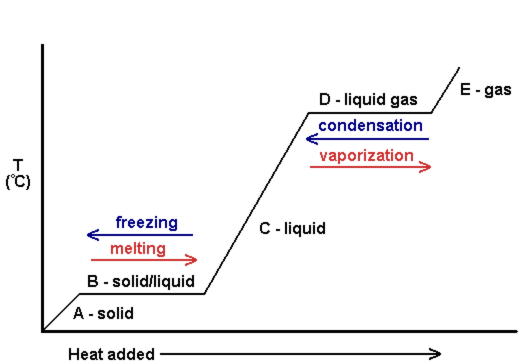 |
| http://www.kentchemistry.com/images/links/matter/HeatCool.gif Things to Know about Heating/Cooling:
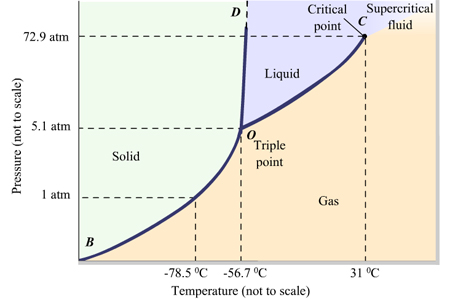
Phase Diagram: A representation of the states of a substance in relation to temperature and pressure. |
 |
| http://d2vlcm61l7u1fs.cloudfront.net/media%2F654%2F654abc63-5ebf-4f33-9507-c38acd719f38%2FphpAsNMSr.png |
Things to Know about Phase Diagrams:
- triple point-point at which the solid, liquid, and vapor states all have the same vapor pressure and coexist.
- critical temperature-the temperature above which the vapor cannot be liquefied, regardless of the pressure applied.
- critical pressure-the pressure required to liquefy the vapor at the critical temperature
- critical point-point at which the critical temperature and critical pressure coincide
Useful Links:
This a quite an amazing and expansive resource of information you've build up here! Will definite use this to study!
ReplyDelete