Blog Post #1
Earlier this week, we had started a new unit over Energy and Phase Changes.
Energy changes accompany all chemical reactions and are due to the rearranging of chemical bonds. In fact, addition of energy is always a requirement for the breaking of bonds, but the breaking of bonds in itself, does not release energy.
Chemists define work as directed energy change resulting from a process.
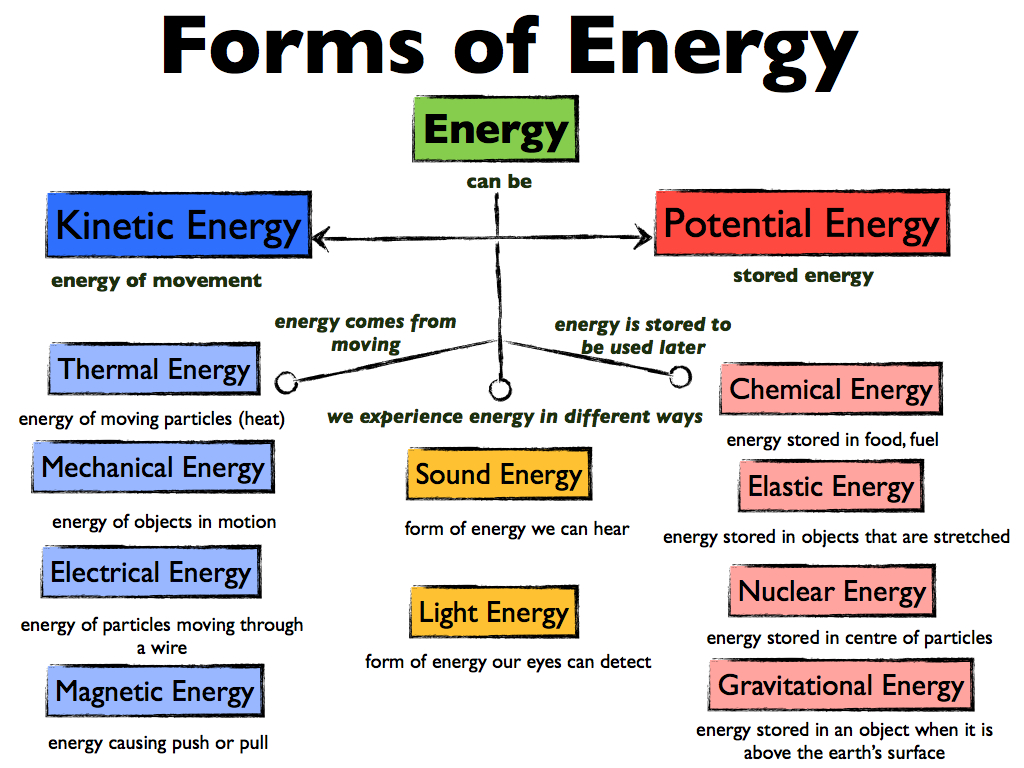
There are many different types of energy:
Kinetic Energy- energy of motion
Radiant Energy- Energy from the sun or solar energy
Thermal Energy-Energy associated with the random motion of atoms and molecules
Chemical Energy- Energy stored within the structural units of chemical substances
Potential Energy- Energy stored or energy of position
Earlier this week, we had started a new unit over Energy and Phase Changes.
Energy changes accompany all chemical reactions and are due to the rearranging of chemical bonds. In fact, addition of energy is always a requirement for the breaking of bonds, but the breaking of bonds in itself, does not release energy.
Chemists define work as directed energy change resulting from a process.
There are many different types of energy:
Kinetic Energy- energy of motion
Radiant Energy- Energy from the sun or solar energy
Thermal Energy-Energy associated with the random motion of atoms and molecules
Chemical Energy- Energy stored within the structural units of chemical substances
Potential Energy- Energy stored or energy of position
 |
| http://mskuksclass.weebly.com/uploads/1/9/7/1/19719395/8207568_orig.jpg In calculating heat, we typically use the formula Q=mcAt Q=heat in joules M=mass in grams c=specific heat in (J/g degree C) AT=Change in temperature  https://s-media-cache-ak0.pinimg.com/736x/cd/80/5e/cd805e4fe12071be7b4a7ce04fac0709.jpg Useful Links: How To Determine Specific Heat Measuring the Quantity of Heat |
I have the formula -mcat=mcat burned into my head! Can never get it out now. It is extremely useful in this unit though!
ReplyDeleteThank you
ReplyDelete